A radically-new science-enabled technology
The main technological objective, which entails a paradigm shift for neuronal drug screening assays, consists of physically and functionally connecting cultured biological neurons with simulated neural circuits, through nanowire (NW) connections that are distributed at multiple subcellular locations, alike real synapses. The biological and simulated networks form a closed-loop, trainable and adaptive system.
We aim at a disruptive system by reproducing, in vitro, both subcellular and network dysfunctions to closely match real brain neuropathologies.
Our final target is to validate the system, by evaluating the effect of drugs for Alzheimer’s Disease (AD) on human iPSC (induced Pluripotent Stem Cell) derived neurons. Towards this goal, our Specific Objectives are:
- O1. The Synaptors: to engineer –inspired by brain’s electrical synapses9– NW-mediated contacts between simulated and cultured neurons, to evoke local responses resembling natural postsynaptic potentials. The technology relies on NWs with functionalized surface, operated to depolarize subcellular compartments of neurons, in a selective, localized manner.
- O2. The AlzModel: to build a detailed, multiscale, neuronal network model that reproduces key deficits of AD (e.g. impaired synaptic plasticity/rigidity, decreased NMDA currents, dendritic atrophy etc.). The model relies on realistic incorporation of inhibitory and excitatory neurons, their connectivity and physiological characteristics of the pathology.
- O3. Human AD model on Synaptors: to establish a realistic in vitro model of AD, cultured on top of the vertical NWs. We will use iPSC- derived human neurons exhibiting the pathology induced by AD patient brain extracts. AD involves synapse dysfunction (loss of spines, NMDA receptors, synapse rigidity etc.) followed by dendritic atrophy that eventually leads to neuronal death10,11. Thus, access and control of the subcellular compartments of diseased neurons is critical for drug discovery.
- O4. The NEUREKA hybrid platform: to establish a controllable hybrid system, able to drive activity to reproduce in-brain pathology. AlzModel neurons connect through Synaptors to cultured ones, while recording NWs feed back to the model, thus completing a dynamic, self-adaptive closed-loop.
- O5. The NEUREKA drug testing PoC: to evaluate the ability of selected compounds to restore synaptic, cellular and/or network function in the cultured neurons. NEUREKA is benchmarked against state-of-the art technologies used in the pharma industry (e.g. calcium imaging-based screening).
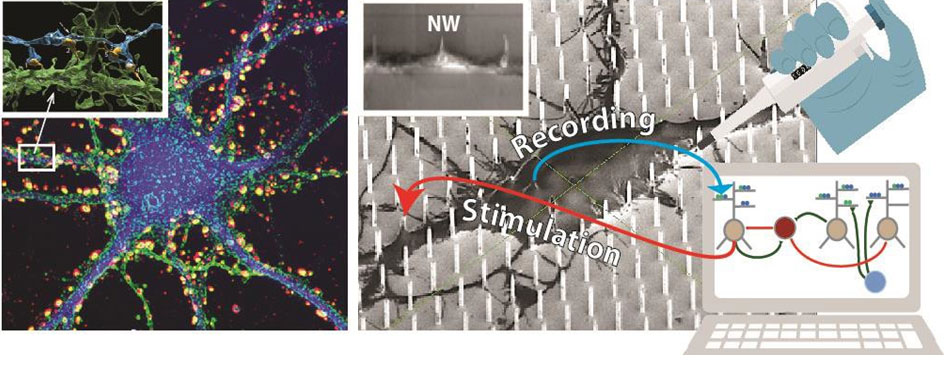
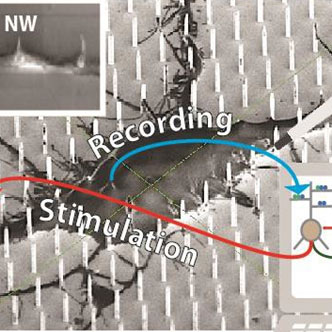
Left. Cultured cortical neuron stained for synapses. Synaptic boutons are distributed all over (soma/dendrites).
Inset: 3D reconstruction of nanoscale synaptic contacts (yellow) formed by an axonal branch (blue) and a dendrite (green).
Right. Sketch representing the NEUREKA hybrid concept. Disease neurons are cultured on a high resolution MEA extended to incorporate vertical nanowires (NWs) that establish intimate contacts at multiple subcellular locations (scanning electron microscopy micrograph in background). NWs are used to stimulate dendrites, thus emulating synaptic inputs, and to read out somatic responses. A biophysical network model, calibrated to reproduce Alzheimer’s physiology (e.g. having increased LTP threshold and rigid synapses), drives the biological network through NWs in an adaptive closed-loop. Drug screening is performed via application of compounds onto cultured neurons (symbolized by the pipette) and assessment of electrophysiological effects throughout the network with NWs. Inset: dendritic branch engulfing three NWs